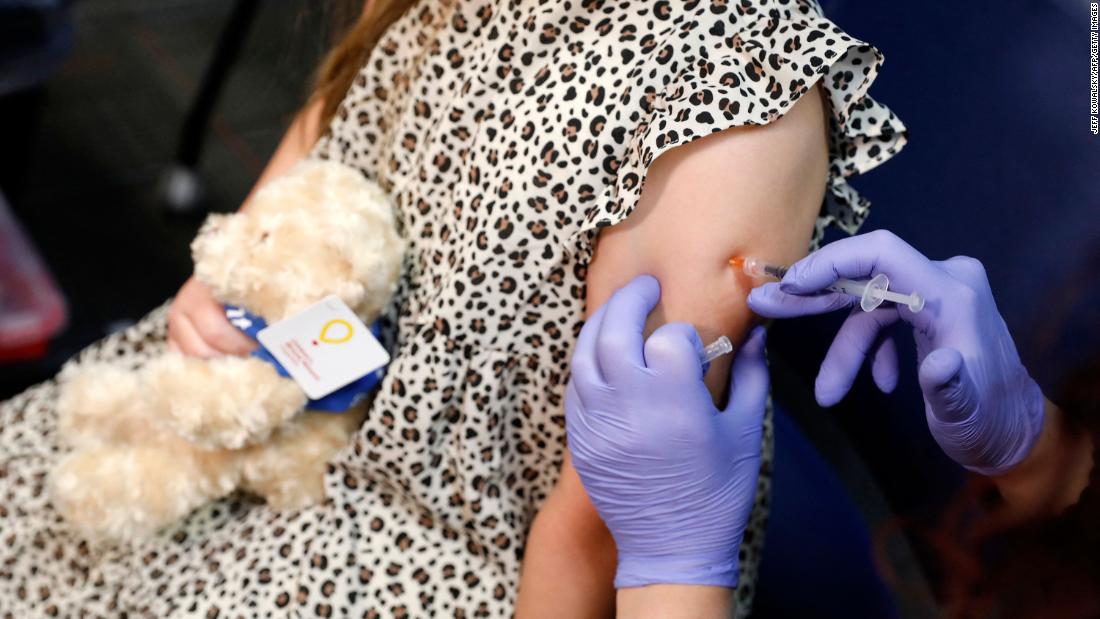
The antibody levels tested one month after the third dose showed that the vaccine provided an immune response similar to the two doses aged 16 to 25 years, the two companies said.
Mid-term results showed that the vaccine efficacy against symptomatic Covid-19 was 80.3% in this youngest age group. Both companies identified 10 symptomatic cases at least 7 days after the third dose. However, efficacy rates will not be determined until at least 21 symptomatic cases have been found in the vaccine group and compared to the number of symptomatic cases in the placebo group.
Vaccines in this youngest age group are smaller than those used in older groups. People over the age of 12 will be vaccinated twice with 30 micrograms, and children aged 5 to 12 will be vaccinated twice with 10 micrograms. Both of these groups are eligible for booster immunization.
For children 6 months to 5 years, the Pfizer / BioNTech vaccine is given in 3 doses of 3 micrograms. The first two doses were given at 3-week intervals, and the third dose was given at least 2 months after the second dose.
“These top-line safety, immunogenicity, and efficacy data are promising and, subject to regulatory approval, in the hope that this vaccine will be available to young children as soon as possible, worldwide. We look forward to completing the submission to the regulatory authorities in the near future, “Pfizer Chairman and CEO Albert Bourla said in a statement last month.
Dr. William Schaffner, a professor of infectious diseases at Vanderbilt University School of Medicine, said vaccine scientists are carefully adjusting doses for young children to “minimize side effects and have good results.” Said.
“We consider this a three-dose vaccine. Preliminary data from the Omicron era show that it’s actually 80% effective,” Schaffner said. “We would want to look at it very carefully, but for now, that’s good news.”
In the United States, children under the age of 5 are the only age group not eligible for vaccination against Covid-19. Vaccines in this group were postponed because the results of the two-dose Pfizer / BioNTech vaccine series did not show the expected level of protection. The two companies said they would modify the study to add a third dose.
In February, the U.S. Food and Drug Administration requested companies to submit an emergency use authorization application based on double-dose data, but held a meeting of the Vaccine Advisory Board to review the third-dose data. It was postponed.
The FDA will convene a Vaccine and Related Biopharmaceutical Advisory Committee on June 14 to discuss Moderna’s Covid-19 vaccine EUA requirements for people aged 6 to 17 years.
On June 15, the Commission will discuss the EUA’s request for infant vaccines by Moderna and Pfizer.
Moderna submitted vaccine data to the FDA in late April for children aged 6 months to 5 years. The submission is based on two 25 microgram doses given at 28-day intervals.
Source: www.cnn.com
